Aeration and agitation are very important processes in fermentation, especially in aerobic fermentation where microorganisms require oxygen to grow and produce desired products.
Bioreactor Aeration:
Table of Contents
ToggleOxygen is essential for the growth and metabolism of aerobic microorganisms. During aerobic fermentation, oxygen is needed to support the growth of aerobic microorganisms like yeast, bacteria, and fungi. Aeration ensures that an adequate supply of oxygen is available for the microorganisms to carry out their metabolic processes efficiently.
Bioreactor Agitation:
Agitation involves the mixing or stirring of the fermentation broth. It helps in several ways:
- Uniform Distribution of Nutrients: Agitation ensures that nutrients, including sugars, minerals, and other growth factors, are evenly distributed throughout the fermentation broth. This helps in promoting uniform growth of microorganisms and ensures consistent product quality.
- Prevention of Shear Stress: While agitation is important, excessive shear stress caused by high-speed agitation can damage fragile microbial cells, affect product yield, and reduce fermentation efficiency. Thus, the level of agitation must be optimized to maintain an appropriate balance between mixing efficiency and shear stress.
- Removal of CO2: During fermentation, microorganisms produce carbon dioxide (CO2) as a byproduct. Agitation helps in removing CO2 from the fermentation broth, preventing its accumulation, which can inhibit microbial growth and fermentation rates.
- Heat Transfer: Agitation facilitates heat transfer within the fermentation broth. Microbial metabolism generates heat, which can elevate the temperature of the fermentation vessel. Effective agitation helps in dissipating heat and maintaining optimal temperature conditions for microbial growth and metabolic activity.
Oxygen Transfer Rate (OTR):
Oxygen Transfer Rate (OTR) is fundamental in aerobic fermentation processes where microorganisms require oxygen for growth and metabolic activities. OTR refers to the rate at which oxygen is transferred from the gas phase (e.g., air) into the liquid phase (fermentation broth) within a bioreactor or fermenter. It is a critical parameter because it directly influences the growth, productivity, and efficiency of aerobic microorganisms during fermentation.
Several factors affect the oxygen transfer rate:
- Agitation and Mixing: Effective agitation and mixing are essential to maintain a high oxygen transfer rate. Agitation ensures the dispersion of oxygen bubbles throughout the fermentation broth, facilitating contact between oxygen molecules and microbial cells. Proper mixing prevents the formation of stagnant zones where oxygen transfer may be limited.
- Surface Area and Bubble Size: The surface area available for oxygen transfer and the size of oxygen bubbles significantly influence the oxygen transfer rate. Increasing the surface area of the gas-liquid interface by using smaller bubbles or increasing the agitation intensity enhances oxygen transfer efficiency.
- Gas Flow Rate: The rate at which oxygen-containing gas (usually air or oxygen-enriched air) is supplied to the bioreactor affects the oxygen transfer rate. Higher gas flow rates can increase the oxygen transfer rate, but excessive gas flow may lead to inefficient utilization of oxygen and increased energy consumption.
- Physical Properties of the Fermentation Broth: The physical properties of the fermentation broth, such as viscosity, density, and surface tension, influence oxygen transfer. Higher viscosity and surface tension can hinder oxygen transfer by impeding bubble formation and dispersion.
- Temperature and Pressure: Temperature and pressure conditions within the bioreactor affect oxygen solubility and gas-liquid mass transfer coefficients, which impact oxygen transfer rates. Generally, lower temperatures and higher pressures improve oxygen solubility but may increase energy consumption.
- Vessel Design and Geometry: The design and geometry of the fermentation vessel play a significant role in determining the oxygen transfer rate. Factors such as vessel shape, baffles, sparger design, and presence of foam can affect oxygen distribution and transfer efficiency.
Measurement and optimization of oxygen transfer rate are crucial for maximizing the productivity and yield of aerobic fermentation processes. Techniques such as mass balance calculations, oxygen uptake rate measurements, and oxygen saturation monitoring are commonly employed to assess and optimize oxygen transfer in bioreactor systems. By understanding and controlling the factors that influence oxygen transfer rate, bioprocess engineers can enhance the performance and scalability of aerobic fermentation processes for various industrial applications, including pharmaceuticals, biotechnology, food, and beverage production.
OTR=KLa(C∗−C)
Where:
- OTR is the Oxygen Transfer Rate.
- KLa is the overall volumetric mass transfer coefficient, representing the efficiency of oxygen transfer in the system. It accounts for factors such as agitation, gas flow rate, vessel design, and physical properties of the fermentation broth.
- C∗ is the equilibrium concentration of dissolved oxygen in the fermentation broth, typically measured under saturation conditions.
- C is the actual concentration of dissolved oxygen in the fermentation broth.
The KLa term can be further broken down into components such as the gas-liquid mass transfer coefficient (kL), the interfacial area (a), and the driving force for oxygen transfer. These components are influenced by factors like agitation speed, gas flow rate, temperature, pressure, and the physical properties of the fermentation broth.
It’s important to note that accurately estimating OTR requires experimental determination of parameters like KLa, which can vary depending on the specific conditions and characteristics of the fermentation system.
Methods to determine KLa:
There are several methods used to determine the overall volumetric mass transfer coefficient (KLa) in bioreactor systems. These methods provide valuable insights into the efficiency of oxygen transfer and help optimize fermentation processes. Some common methods include:
a) Sulphite Oxidation technique
b) Gassing out technique:
i)Static method of Gassing out
ii) Dynamic method
c) Off-Gas Analysis Method
d) Empirical Correlations
a) Sulphite Oxidation technique: OTR is determined by the oxidation of Sodium sulphite Solution.
This technique depends on the rate of conversion of a 0.5 M solution of Sodium sulphite to Sodium sulphate in the presence of a copper or cobalt catalyst.
Na2SO3+ 0.5 O2 = Na2SO4
As much oxygen enters the solution, oxygen is immediately consumes in the oxidation of sulphite, So that the sulphite oxidation rate is equivalent to the Oxygen- transfer rate.
b) Static Method of Gassing out: In this techniques, the concentration of O2 is decreased to almost zero by using nitrogen gas. So that the solution is free from oxygen (deoxygenated). The deoxygenated liquid is then aerated and agitated and then increased in DO monitored using DO probe.
The increased in DO concentration calculated by
dCL/dt = KLa (CL*– CL)
Where, dCL/dt = OTR
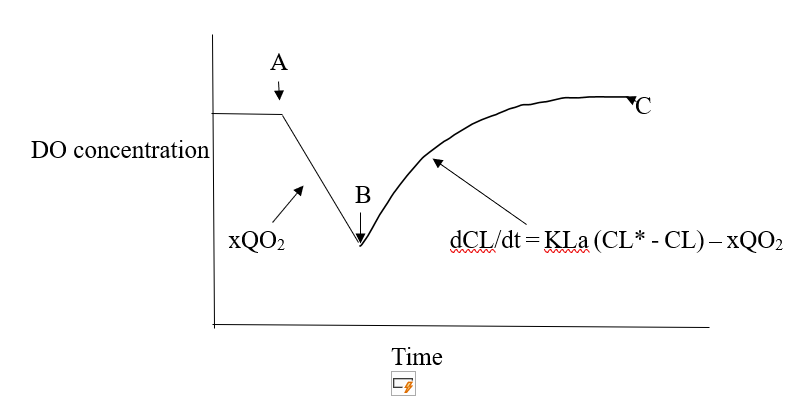
Dynamic Gassing Out Method: In this method, air supply to the fermentation is stopped which results in a linear decline in the DO concentration due to the respiration of the culture. The slope of the line AB is a measure of the respiration rate of the culture.
Now at point B, the aeration is resumed then the DO concentration gradually increases. Over the period, BC, the observed increased DO concentration is the difference between the transfer of oxygen into solution & the uptake of O2 by the respiring cultures as expressed by below equation:
dCL/dt = KLa (CL* – CL) – xQO2
Where, x =Concentration of Biomass
xQO2 = Specific respiration rate (mmoles of O2 / g biomass / h)
c) Off-Gas Analysis Method: This method involves analyzing the composition of the off-gas exiting the bioreactor. By measuring the concentrations of oxygen and other gases in the off-gas stream, along with the gas flow rate, it is possible to calculate the rate of oxygen transfer from the gas phase to the liquid phase. The KLa value can then be determined using mass balance equations and gas-liquid equilibrium principles.
d) Empirical Correlations: Empirical correlations based on experimental data and operating conditions are often used to estimate KLa These correlations relate KLa to parameters such as agitation speed, gas flow rate, vessel geometry, and physical properties of the fermentation broth. While empirical correlations provide quick estimates of KLa, they may not capture the full complexity of the mass transfer process.
Factor affecting KLa values in fermentation vessels:
The overall volumetric mass transfer coefficient (KLa) in fermentation vessels is influenced by several factors, including:
- Agitation Intensity: Agitation plays a crucial role in promoting gas-liquid mass transfer by enhancing the dispersion of gas bubbles in the liquid phase. Higher agitation intensities lead to increased turbulence and improved gas-liquid contact, resulting in higher KLa
- Gas Flow Rate: The rate at which gas (typically air or oxygen) is sparged into the fermentation vessel affects KLa. Higher gas flow rates increase the number of gas bubbles entering the liquid phase, thereby enhancing oxygen transfer. However, excessively high gas flow rates may lead to gas bypassing and inefficient utilization of oxygen.
- Vessel Design and Geometry: The design and geometry of the fermentation vessel influence KLa by affecting gas-liquid contact and flow patterns. Factors such as impeller design, baffle configuration, vessel shape, and aspect ratio can impact the distribution of gas bubbles and the efficiency of oxygen transfer.
- Physical Properties of the Fermentation Broth: The physical properties of the fermentation broth, including viscosity, density, surface tension, and foaming tendency, influence KLa. Higher viscosity and surface tension can hinder gas-liquid mass transfer by impeding bubble formation and dispersion.
- Temperature and Pressure: Temperature and pressure conditions within the fermentation vessel affect gas solubility and mass transfer coefficients, which in turn influence KLa. Lower temperatures generally increase gas solubility but may also decrease mass transfer rates. Pressure changes can alter gas solubility and affect bubble size and distribution.
- Microbial Activity: Microbial metabolism and growth within the fermentation broth can impact KLa by altering oxygen consumption rates and gas-liquid mass transfer dynamics. Higher microbial activity may deplete oxygen more rapidly, leading to increased oxygen demand and potentially lower KLa
- Foam Formation: Foam formation in the fermentation vessel can interfere with gas-liquid contact and gas transfer efficiency. Foam may trap gas bubbles, reduce surface area available for mass transfer, and impede oxygen transfer. Effective foam control strategies are necessary to maintain optimal KLa
- pH and Nutrient Levels: pH and nutrient levels in the fermentation broth can influence microbial growth and metabolic activity, thereby affecting oxygen consumption rates and oxygen demand. Optimal pH and nutrient conditions are important for maximizing KLa and overall fermentation performance.
By understanding and optimizing these factors, bioprocess engineers can enhance oxygen transfer rates and improve the efficiency and productivity of fermentation processes for various industrial applications, including pharmaceuticals, biotechnology, food, and beverage production.
Relationship between KLa and power consumption:
The power consumption in a fermentation vessel primarily comes from agitation, which is achieved through the rotation of impellers or stirrers. The energy input from agitation contributes to the dispersion of gas bubbles and promotes gas-liquid mass transfer, thus influencing KLa.
The relationship between KLa and power consumption can be approximated by the following equation:
![]()
Where,
- KLa represents the mass transfer coefficient, which measures the effectiveness of transferring a gas (usually oxygen) from the gas phase into a liquid phase in a bioprocess.
- P typically denotes the power input to the bioreactor, which influences the rate of oxygen transfer.
- V represents the volume of the liquid (usually the volume of the bioreactor), which affects the mass transfer rate.
- k is a proportionality constant.
- x and y are exponents that determine the relationship between P, V, and KLa.
- Vs might denote the superficial gas velocity, which is the velocity of the gas in the bioreactor.
The equation suggests that the mass transfer coefficient (KLa) depends on the power input per unit volume (P/V) raised to the power of x, and the volume (V) raised to the power of y, with k being a proportionality constant.
This equation is fundamental in designing and optimizing bioprocess systems, especially in bioreactors where the efficient transfer of gases like oxygen is crucial for biological processes such as fermentation or cell culture.
Relation between power consumption and operating variables:
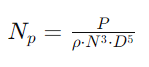
Where:
- P is the power input to the fluid (in watts or horsepower).
- ρ is the fluid density (in kg/m³ or lb/ft³).
- N is the rotational speed of the agitator (in revolutions per second or per minute).
- D is the diameter of the impeller or agitator (in meters or feet).
- Np is the power number
Power Number: The power number (Np) is a dimensionless parameter used in fluid dynamics to characterize the power consumption of an agitated system relative to the fluid properties and the size and geometry of the agitator. It describes the relationship between the power input to the fluid (typically through agitation) and the fluid’s flow characteristics.
The power number essentially represents the ratio of the power input to the fluid to the dynamic forces exerted by the fluid flow. A high power number indicates that a significant portion of the power input is used to overcome fluid inertia and viscous forces, resulting in more vigorous mixing and agitation.
The power number is often used in conjunction with other dimensionless parameters, such as the Reynolds number (Re) and the Froude number (Fr), to characterize and compare different mixing systems and to scale up processes from laboratory-scale to industrial-scale equipment.
Understanding the power number helps engineers and researchers optimize mixing processes, select appropriate agitator designs, and predict power consumption in various fluid mixing applications, including chemical reactors, fermenters, and industrial mixing vessels.
Reynold Number: The Reynolds number (Re) is a dimensionless parameter used in fluid dynamics to characterize the flow regime of a fluid and predict its behavior. It represents the ratio of inertial forces to viscous forces within the fluid flow and helps classify flow patterns as either laminar or turbulent.
The Reynolds number is defined as:
![]()
Where:
- ρ is the density of the fluid (in kg/m³).
- v is the velocity of the fluid (in m/s or ft/s).
- L is a characteristic length scale of the flow (such as diameter or hydraulic radius) (in m or ft).
- μ is the dynamic viscosity of the fluid (in Pa·s or lb·s/ft²).
The Reynolds number helps predict the transition from laminar to turbulent flow. In general:
- For <2000Re<2000, the flow is considered laminar, characterized by smooth, orderly flow patterns with minimal mixing and low levels of turbulence. In laminar flow, the fluid moves in parallel layers, and mixing between layers is limited.
- For >4000Re>4000, the flow is considered turbulent, characterized by chaotic, irregular flow patterns with high levels of mixing and turbulence. In turbulent flow, eddies and vortices develop, enhancing mixing and promoting heat and mass transfer.
- For 2000<<40002000<Re<4000, the flow regime is transitional, exhibiting characteristics of both laminar and turbulent flow. The transition from laminar to turbulent flow depends on factors such as flow velocity, fluid viscosity, and the geometry of the flow system.
The Reynolds number is widely used in fluid mechanics and engineering to analyze and design various fluid flow systems, including pipes, channels, pumps, turbines, and mixing equipment. By understanding the Reynolds number, engineers can predict flow behaviors, optimize system performance, and ensure efficient operation of fluid flow processes.
Fluid Rheology:-
Fluid rheology is the study of how fluids flow and deform under different conditions. It encompasses the study of the physical properties of fluids, including viscosity, elasticity, and flow behavior. These properties are essential for understanding and predicting the behavior of fluids in various industrial, scientific, and engineering applications.
Here are the key concepts and equations related to fluid rheology, presented in a simplified form:
- Viscosity (η):
- Viscosity is a measure of a fluid’s resistance to flow. It quantifies how easily a fluid deforms under an applied shear stress.
- The viscosity of a fluid is often represented by the symbol η.
- Newton’s Law of Viscosity describes the relationship between shear stress (τ) and shear rate (γ):
τ=η⋅γ
- In simple terms, this equation states that the shear stress experienced by a fluid is directly proportional to the shear rate and viscosity of the fluid. The constant of proportionality is the viscosity.
- Newtonian Fluids:
- Newtonian fluids exhibit a linear relationship between shear stress and shear rate, as described by Newton’s Law of Viscosity.
- Examples of Newtonian fluids include water, air, and most common liquids and gases.
- Non-Newtonian Fluids:
- Non-Newtonian fluids do not follow Newton’s Law of Viscosity and may exhibit complex flow behavior.
- There are different types of non-Newtonian fluids, including:
- Shear-Thinning: Viscosity decreases as shear rate increases. Examples include ketchup, paint, and some polymer solutions.
- Shear-Thickening: Viscosity increases as shear rate increases. Examples include cornstarch suspensions and some colloidal solutions.
- Viscoelastic: These fluids exhibit both viscous and elastic behavior. Examples include certain polymer melts and biological fluids like blood.
- Elasticity (G):
- Elasticity refers to a fluid’s ability to deform and return to its original shape when the applied stress is removed.
- The elastic modulus, often denoted as G or ′G′, measures a fluid’s resistance to deformation.
- For viscoelastic fluids, the storage modulus (′G′) represents elastic behavior, while the loss modulus (′′G′′) represents viscous behavior.
- Flow Behavior:
- Fluids can exhibit different flow behaviors under applied forces, such as:
- Newtonian Flow: Obeys Newton’s law of viscous flow. Where Shear stress is directly proportional to shear rate. Thus, a Newtonian fluid has a constant viscosity regardless of shear, so that the viscosity of Newtonian fermentation broth will not vary with agitation rate.
- Non-Newtonian Flow: Does not obey Newton’s law of viscous flow. Shear stress is not directly proportional to shear rate, leading to various flow patterns and behaviors. Thus the viscosity of a Non-Newtonian fermentation broth will vary with agitation rate.
- Fluids can exhibit different flow behaviors under applied forces, such as:
Bioreactor VVM:-
VVM stands for “Volume of air per unit volume of liquid per minute.” It is a critical parameter in bioprocess engineering used to measure the efficiency of oxygen transfer into the liquid medium within a bioreactor. VVM is a key factor in determining the oxygenation levels required for aerobic microbial growth in various biotechnological processes such as fermentation and cell culture.
- Definition: VVM represents the ratio of the volume of air (or gas) supplied to the bioreactor per unit volume of liquid in the bioreactor per minute. It is typically expressed in units of reciprocal minutes (1/min).
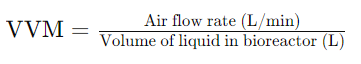
- Importance: VVM is crucial because it directly influences the rate at which oxygen is transferred from the gas phase to the liquid phase within the bioreactor. Adequate oxygenation is essential for the growth and metabolism of aerobic microorganisms, such as bacteria, yeast, and certain types of cells.
- Optimization: Bioprocess engineers often optimize VVM to achieve the desired oxygen transfer rate based on the specific requirements of the microorganisms being cultured and the process conditions. Too low a VVM may result in inadequate oxygenation, leading to reduced growth rates or cell death, while too high a VVM can cause excessive foaming and increased energy consumption.
- Control: VVM can be controlled by adjusting the flow rate of the air or gas supplied to the bioreactor, as well as by modifying the agitation speed and design of the aeration system.
- Monitoring: Monitoring VVM during bioreactor operation helps ensure that optimal oxygen levels are maintained throughout the fermentation or cell culture process. This is often done using online sensors or probes that measure dissolved oxygen concentrations in the liquid medium.
In summary, Bioreactor VVM is a critical parameter in bioprocess engineering that helps optimize oxygen transfer rates and ensure optimal growth conditions for aerobic microorganisms in bioreactor systems.

