Easy Guide to Installing and Commissioning a Fermenter for API Production
Table of Contents
ToggleMaking Active Pharmaceutical Ingredients (APIs) using microbes like bacteria or yeast is a key process in the pharma industry. Setting up a fermenter for this purpose isn’t just about buying equipment—it involves proper planning, designing, testing, and following strict quality standards.
This guide explains the full journey of installing and commissioning a fermenter for API production in simple language.
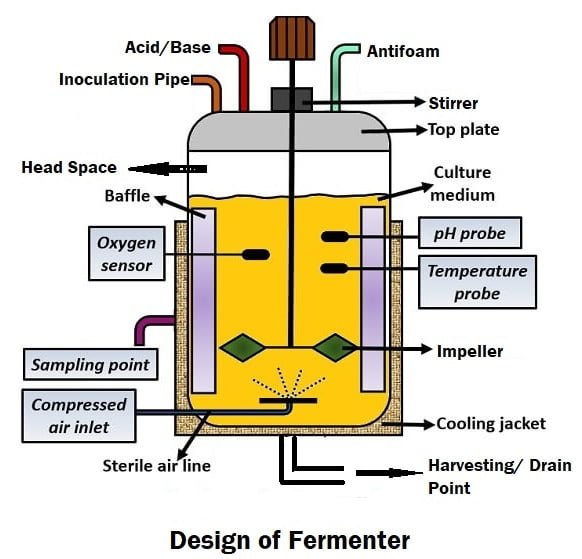
1. Start with Understanding What You Need:
Before buying anything, it’s important to know exactly what you’re trying to make and how much you need to produce. This helps you choose the right fermenter and set up everything correctly.
Things to Think About:
- Type of API: Are you using bacteria, fungus, or yeast?
- Fermentation Process: Will it be batch, fed-batch, or continuous?
- Scale of Production:
- Small scale (10–100 L)
- Medium/clinical scale (100–1000 L)
- Large/commercial scale (up to 20,000 L)
- How much product is expected per batch?
- Which regulations apply? (FDA, WHO-GMP, etc.)
2. Choosing the Right Fermenter:
Your fermenter should be built in a way that helps microbes grow efficiently, safely, and under full control.
Key Features of a Good Fermenter:
- Size: Depends on your production needs.
- Material: Stainless steel (SS316L) is best for cleanliness and durability.
- Mixing System: Helps keep everything uniform inside.
- Temperature Control: Using a double-layered jacket or coils.
- Air Supply: Includes air filters, sparger, and baffles for proper oxygen.
- Sensors: For pH, oxygen, temperature, foam, and level.
- Automation: Control system (like PLC or SCADA) for easy monitoring.
- Sterilization (SIP): Built-in steam system for sterilizing parts.
- Cleaning (CIP): Spray balls and automatic cleaning cycles.
3. Designing the Facility:
The fermenter must be placed in a clean, organized, and well-ventilated space that meets pharma standards.
Key Facility Design Tips:
- Clean Zones:
- Grade D: Media preparation.
- Grade C or B: Fermentation and inoculum preparation.
- One-Way Movement: People and materials should not cross paths.
- Cleanroom Design:
- Walls and ceilings should be smooth and easy to clean.
- Floors should be slip-proof and chemical-resistant.
- Air Handling (HVAC): HEPA filters, proper airflow, and temperature/humidity control.
4. Equipment You Need Before and After Fermentation:
Before Fermentation (Upstream):
- Media Tank: For preparing and sterilizing nutrients.
- Seed Fermenter: Smaller tank for growing microbes before transferring to the main fermenter.
- Feed Tanks: For adding nutrients during the run.
- Air Handling System: Clean, dry, and oil-free air.
- CIP/SIP System: For cleaning and sterilizing equipment.
After Fermentation (Downstream):
- Harvest Tank: Collects the fermented product.
- Filtration or Centrifuge: Separates cells from the liquid.
- Purification Units: Chromatography and filtration for cleaning the product.
- Dryers: Turns the liquid product into powder.
5. Utility Systems (Support Systems):
These are essential to run everything smoothly and safely.
Main Utilities:
- Clean Steam: For sterilizing equipment.
- Chilled Water or Glycol: For keeping the temperature under control.
- Compressed Air: Should be clean anddry.
- Vacuum System: Needed during drying and filtration.
- Purified Water (PW) or Water for Injection (WFI): For cleaning and making product solutions.
- Effluent Treatment Plant (ETP): For handling waste.
6. Automation and Control:
Using automation helps maintain product quality and keeps the process under control.
What You Need:
- SCADA/PLC System: For controlling and monitoring.
- Alarms: To alert you when something goes wrong.
- Data Recording: Software that follows 21 CFR Part 11 rules.
- Remote Access (Optional): To monitor things even when off-site.
7. Documentation and SOPs:
In pharmaceutical manufacturing, “If it’s not written down, it didn’t happen.” You must document everything properly.
Important Records:
- Standard Operating Procedures (SOPs)
- Batch Records (BMR)
- Cleaning & Sterilization Logs
- Calibration & Maintenance Records
- Environmental Monitoring Reports
8. Project Timeline (From Start to Finish):
Step | Time Needed |
Requirement & Design | 1–2 months |
Buying & Manufacturing Equipment | 2–4 months |
Delivery & Setup | 1–2 months |
Testing & Validation | 2–3 months |
Submitting to Regulatory Body (if needed) | 1–2 months |
9. Testing and Validating the System:
After installation, you must test the fermenter and confirm it works properly and meets GMP standards.
9.1 Before Testing On-Site:
- URS (User Requirements): What you expect the system to do.
- FDS (Design Document): How the system will meet your needs.
- Risk Assessment: Identify possible failures and how to handle them.
9.2 Factory Acceptance Test (FAT):
- Happens at the vendor’s site.
- Checks equipment quality, control panel, and instruments.
9.3 Site Acceptance Test (SAT):
- Done after installation at your site.
- Checks connections, cleaning system, and controls.
9.4 Qualification Steps:
- DQ (Design Qualification): Verifies design meets your needs.
- IQ (Installation Qualification): Confirms proper installation.
- OQ (Operational Qualification): Checks function and control system.
- PQ (Performance Qualification): Real fermentation runs to confirm everything works as expected.
9.5 Final Validations:
- Cleaning Validation: Make sure cleaning removes all residues and microbes.
- Sterilization Validation: Use test organisms to confirm sterilization.
- Calibration Plan: Schedule for checking all instruments.
- Maintenance Plan: Regular upkeep of pumps, valves, and sensors.
10. Final GMP Documentation Package:
Keep everything organized and ready for audits:
- URS, FDS, Risk Assessment reports
- DQ, IQ, OQ, PQ records
- FAT/SAT reports
- Equipment manuals and certificates
- SOPs
- Cleaning, calibration, and monitoring logs
Conclusion:
Installing and commissioning a fermenter for API production is not just a technical task—it’s a step-by-step project that requires planning, teamwork, and attention to detail. When done properly, it ensures high product quality, regulatory approval, and smooth long-term operations.
With the right system and procedures in place, you’ll have a robust and compliant setup that supports successful API manufacturing for years to come.

