What is Microbial Growth?
Table of Contents
ToggleMicrobial growth is the process by which microorganisms, such as bacteria, fungi, and viruses, multiply and proliferate in a given environment. This phenomenon plays a vital role in various industries, including pharmaceuticals, food production, and environmental science. Microbial growth is a result of both cell division and increases in cell size. Understanding microbial growth kinetics is crucial for optimizing processes, ensuring product safety, and combating infectious diseases.
In a suitable nutrient medium, microorganisms extract nutrients from the medium and some parts from the nutrients are used for energy production & some parts are used for biosynthesis and products formation. Microbial growth mainly depends on the ability of the cell to form new protoplasm from nutrients available in the environment.
The Factors Influencing Microbial Growth
Microbial Growth Kinetics
Before understanding the kinetics of microbial growth, lets understand the microbial growth phases.
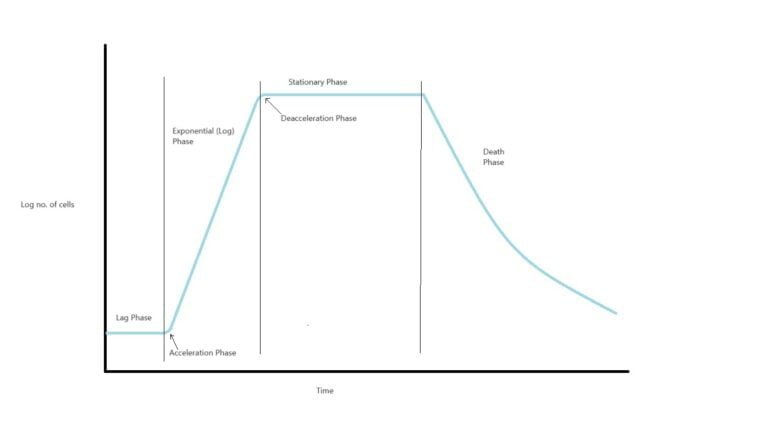
Understanding Growth Phases (Growth curve):
Microbial growth is a dynamic process that can be divided into several distinct phases. Each phase is characterized by specific changes in cell population, metabolic activity, and growth rate. Understanding these phases is fundamental for various applications, including food production, pharmaceuticals, and environmental management.
Microbes are growing in following culture method: Batch culture method, Fed-batch culture method & Continuous culture method.
Before we look into the specifics of different culture methods, let’s understand the fundamental microbial growth phases:
Lag Phase
The journey begins with the lag phase, where microorganisms acclimate to their environment. During this period, cells prepare for rapid growth by synthesizing enzymes and adjusting to the new conditions. The duration of the lag phase varies depending on factors such as nutrient availability and temperature. Healthy cells transferred from one type of media into the same type of media, with the same environmental conditions, will have the shortest lag period. Damaged cells will have a long lag period because they involve first into repair themselves before they can engage in reproduction.
Note: Multiple Lag phase may sometimes we may be observed when the medium contains more than one carbon source. This phenomenon is known as diauxic growth. It occurs due to shift in metabolic pathways in the middle of a growth cycle. When one carbon source is exhaust, the cells adapt their metabolic activities to utilize the second carbon source.
Exponential (Log) Phase
The exponential phase is marked by rapid cell division. Cells multiply at an exponential rate, resulting in a steep upward curve on growth graphs. This phase is pivotal for biomass production and the synthesis of various metabolites, making it invaluable in biotechnological processes. Since the exponential (log) phase is marked by predictable doublings of the population, where 1 cell become 2 cells, becomes 4, becomes 8 etc. This phase is basically used to mathematically calculate the time it takes for the bacterial population to double in number, known as the generation time (g).
The exponential growth rate is first order reaction,
dX/dt= µ.X
Where, X= Conc. Of microbial cell or cell no.
µ= Specific Growth rate,
t= Time in Hrs.
Stationary Phase
As resources become limited and waste products accumulate, microbial growth enters the stationary phase. Here, cell division slows down, and the net growth rate is zero or the growth rate is equal to the death rate. The stationary phase is vital for understanding the shelf life of products and managing microbial populations in various industries.
Death Phase
In the final stage, the death phase, cells begin to die off at an exponential rate. Factors like nutrient depletion and the accumulation of toxic byproducts contribute to this decline. Understanding the death phase is essential for preserving product quality and controlling unwanted microbial growth.
Kinetics of cell death:
rd= KdN
Where, rd= rate of cell death,
N= no. of viable cells,
Kd= Specific death constant.
Batch Culture: The Basics
Batch culture is the simplest microbial cultivation method. In a batch culture, microorganisms are introduced into a closed system with a fixed volume of growth medium. Where they enters into rapid growth phase by using available nutrients until the medium is depleted or waste products become toxic. Here’s how the growth phases play out in batch culture:
- Lag Phase in Batch Culture: The initial lag phase is marked by slow growth as cells adapt to the new environment. Nutrient availability and initial cell density influence the length of this phase.
- Exponential Phase in Batch Culture: As nutrients are plentiful initially, microorganisms enter the exponential phase, characterized by rapid growth and high metabolic activity.
- Stationary Phase in Batch Culture: Eventually, nutrients are consumed, waste products accumulate, and the stationary phase is reached. Growth slows, and the population stabilizes.
- Death Phase in Batch Culture: In the absence of nutrients, cells enter the death phase. Cell numbers decline rapidly as resources are exhausted.
Effect of Substrate Concentration in Batch Culture:
Batch culture is a commonly used method in laboratories and industries for growing microorganisms, such as bacteria, fungi, and yeast, under controlled conditions. The concentration of the substrate, which serves as the primary nutrient source, plays a significant role in shaping the growth dynamics and overall outcome of the culture.
Substrate Concentration: A Key Factor
The substrate concentration, often referred to as the carbon source or nutrient, is a critical factor that directly influences the growth of microorganisms in batch culture. This substrate is known as the growth rate limiting substrate. It’s typically added in the form of a carbon-containing compound, such as glucose, as it serves as the primary energy source for microbial metabolism.
The Effect of Low Substrate Concentration
- Extended Lag Phase: When the substrate concentration is low, microorganisms enter an extended lag phase. During this phase, they are adapting to the environment and preparing their cellular machinery for growth. This phase can be significantly prolonged when substrate concentrations are limited, as microorganisms need more time to acclimate and activate their metabolic pathways.
- Slower Growth Rate: In low substrate conditions, the growth rate of microorganisms is reduced. They have limited resources to generate energy and build biomass, resulting in slower reproduction and smaller population sizes.
- Lower Biomass Yield: With limited substrate, microorganisms produce less biomass. This can be a critical factor in industrial applications where biomass production is the primary goal.
The Effect of High Substrate Concentration
- Shorter Lag Phase: In contrast, when the substrate concentration is high, microorganisms experience a shorter lag phase. They quickly transition from the adaptation phase to active growth since an abundance of nutrients is available.
- Faster Growth Rate: High substrate concentrations support a faster growth rate. Microorganisms have ample resources to fuel their metabolic processes, resulting in rapid reproduction and larger population sizes.
- Higher Biomass Yield: High substrate concentrations lead to increased biomass production. This is advantageous in applications where the goal is to maximize the yield of microbial biomass for subsequent processes like fermentation or bioproduct production.
Finding the Optimal Substrate Concentration
In batch culture, finding the optimal substrate concentration is a balancing act. Too little substrate can lead to slow growth and limited biomass production, while too much substrate can be wasteful and lead to inefficient resource utilization.
Microbiologists and biotechnologists often conduct experiments to determine the ideal substrate concentration for a specific microorganism and application. This involves testing various substrate concentrations and monitoring growth parameters like biomass yield, growth rate, and metabolic activity.
The specific growth rate is generally found to be a function of 3 parameters: the concentration of growth limiting substrate (S), the maximum growth rate (µmax), and a specific constant (Ks)

Fed-Batch Culture: Optimizing Nutrient Supply
Fed-batch culture is a modification of batch culture designed to prolong the exponential phase. In fed-batch cultures, nutrients are added incrementally to maintain optimal growth conditions. This method allows for higher biomass production and the accumulation of specific products. Here’s how the growth phases unfold in fed-batch culture:
- Lag Phase in Fed-Batch Culture: Similar to batch culture, the lag phase in fed-batch culture involves acclimatization to the environment.
- Exponential Phase in Fed-Batch Culture: Unlike batch culture, the exponential phase in fed-batch culture can be extended by careful nutrient feeding. This leads to higher cell densities and enhanced productivity.
- Stationary Phase in Fed-Batch Culture: Eventually, the stationary phase is reached due to nutrient limitations or other factors, but it occurs at a later stage than in batch culture.
- Death Phase in Fed-Batch Culture: The death phase follows, but again, it is delayed compared to batch culture.
Continuous Culture: Maintaining Steady-State
Continuous culture, also known as chemostat culture, is a method where fresh medium is continuously added to the culture vessel while an equal volume of spent medium is removed. This technique allows for steady-state conditions and is often used in research and industrial applications. The growth phases in continuous culture differ from batch and fed-batch cultures:
- Steady-State in Continuous Culture: Instead of distinct growth phases, continuous culture maintains a steady-state where microbial growth and washout are balanced.
How can we know the exponential phase stated and lag phase finished?
Certainly, we can understand the transition from the lag phase to the exponential phase in microbial growth by observing certain key indicators:
- Growth Curve: One of the primary methods is to monitor the growth curve of the microbial population over time. In the lag phase, there is a relatively slow increase in cell numbers as microorganisms adapt to their environment. When this adaptation phase is complete, you will notice a sudden increase in the rate of growth, resulting in a steep upward curve. This sharp increase signifies the transition into the exponential phase.
- Measuring Biomass: Another way to determine the transition is by measuring biomass. Biomass refers to the total mass of microorganisms in the culture. During the lag phase, the biomass may increase slowly. In the exponential phase, the biomass increases rapidly, and this can be quantified using methods such as optical density measurements or dry cell weight.
- Nutrient Consumption: Microorganisms in the lag phase are adjusting to the available nutrients. As they shift into the exponential phase, they start consuming nutrients more rapidly. Monitoring nutrient consumption rates can help identify the transition point.
- Metabolic Activity: During the lag phase, metabolic activity is lower as cells prepare for growth. In the exponential phase, metabolic activity increases significantly as cells divide rapidly. Measuring parameters like oxygen consumption or carbon dioxide production can provide insights into metabolic changes.
- Cell Division: Microscopic examination of the culture can reveal the transition. In the lag phase, you may observe individual cells or small clusters. In the exponential phase, the population will appear denser as cells divide and form larger colonies.
- Genetic Expression: Analyzing gene expression patterns can also help identify the shift from the lag phase to the exponential phase. During the lag phase, genes related to adaptation and preparation for growth are more active. In the exponential phase, genes associated with cell division and metabolism become more prominent.
- Growth Rate: Mathematically, the growth rate (doubling time) is significantly lower during the lag phase and increases rapidly as the culture enters the exponential phase. By tracking the growth rate, you can pinpoint the transition.
It’s important to note that the exact timing of the transition from the lag phase to the exponential phase can vary depending on factors such as the type of microorganism, the composition of the growth medium, and environmental conditions like temperature and pH. Therefore, a combination of these indicators and regular monitoring is often used to precisely identify when the lag phase has finished and the exponential phase has begun in a microbial culture.
Generation time, also known as doubling time, is a critical concept in microbiology that measures the time it takes for a population of microorganisms to double in number during the exponential growth phase. In simpler terms, it tells us how long it takes for the number of microorganisms in a culture to go from one to two, two to four, four to eight, and so on.
Here’s how you can calculate the generation time:
- Start with a Culture: Begin with a microbial culture at a specific initial population, often denoted as N₀.
- Measure Time: Record the time it takes for the population to double. This is typically done during the exponential growth phase when microorganisms are dividing rapidly.
- Count Doublings: If it takes, for example, 30 minutes for the population to double, you have a generation time of 30 minutes.
Generation time is an essential parameter in microbiology and microbial ecology for several reasons:
- Growth Rate: It provides a measure of how fast a microorganism is growing under specific conditions. Microorganisms with shorter generation times are considered to have faster growth rates.
- Biotechnological Applications: Understanding the generation time is crucial in biotechnology for optimizing the production of various products, such as antibiotics, enzymes, and biofuels.
- Predicting Population Size: Knowing the generation time allows scientists to predict how quickly a microbial population will increase in size under specific conditions.
- Control Strategies: In fields like food safety and healthcare, knowledge of generation time is used to design strategies for controlling and managing microbial populations.
- Environmental Studies: Generation time is used in ecological studies to understand the dynamics of microbial populations in natural environments.
It’s important to note that generation time can vary significantly among different microorganisms and is influenced by factors like temperature, nutrient availability, and other environmental conditions. By calculating and understanding the generation time, scientists can gain insights into the growth characteristics of microorganisms and make informed decisions in various fields, from medicine to industry and environmental science.
How to calculate number of microorganism present in a culture medium?
Calculating the number of microorganisms present in a culture medium involves using the concept of population growth and the initial cell count. The formula you need to use is based on exponential growth. Here’s how to calculate it:
Nt = N₀ * 2^(n/g)
Where:
- Nt is the final number of microorganisms.
- N₀ is the initial number of microorganisms.
- n is the number of generations (doublings) that have occurred.
- g is the generation time (doubling time) in the same units as your time measurement.
Let’s break down the steps to calculate the number of microorganisms:
- Start with the Initial Count (N₀): Begin by determining the initial number of microorganisms in your culture. This could be based on a direct count (e.g., using a hemocytometer or counting colony-forming units) or an estimate.
- Measure the Time (t): Determine the time over which the microbial population has been growing. This time should be in the same units as the generation time (g) you’ll be using.
- Calculate the Number of Generations (n): To calculate the number of generations, divide the total time (t) by the generation time (g). The formula for this is n = t / g.
- Apply the Exponential Growth Formula: Now that you have N₀, n, and g, you can calculate Nt using the formula Nt = N₀ * 2^(n/g). This formula takes into account the initial population, the number of generations, and the generation time to determine the final number of microorganisms.
- Calculate Nt: Plug your values into the formula, and you will get the final number of microorganisms (Nt) present in the culture medium after the specified time.
It’s important to note that this calculation assumes ideal conditions of exponential growth, which may not always apply in real-world scenarios. Factors such as nutrient depletion, competition, and environmental limitations can affect microbial growth. Additionally, accurate measurements of N₀ and generation time are essential for precise calculations.
For practical purposes, especially in laboratory settings, it’s common to use techniques like serial dilution and plating on agar plates to estimate the number of viable microorganisms in a culture. These methods provide a more direct and accurate count of colony-forming units (CFUs), which can be a reliable indicator of microbial population size.
What is the Growth Yield Coefficient?
The growth yield coefficient, Y, is a dimensionless constant that quantifies the efficiency of a microorganism in converting a specific substrate into biomass during its growth. In simpler terms, it tells us how much microbial biomass can be produced from a given amount of substrate. This coefficient is a fundamental parameter used in microbiological and biotechnological research to understand and optimize microbial processes.
Calculating the Growth Yield Coefficient
The growth yield coefficient is typically calculated using the following formula:
Y = ΔX / ΔS
Where:
- Y represents the growth yield coefficient.
- ΔX is the change in microbial biomass (usually measured as the change in cell mass or concentration).
- ΔS is the change in substrate concentration.
In this equation, Y reflects the amount of biomass produced per unit of substrate consumed. It essentially quantifies the efficiency of microbial growth with respect to substrate utilization.
Significance of the Growth Yield Coefficient
Understanding the significance of the growth yield coefficient is crucial in various fields:
1. Bioprocess Optimization
In biotechnological applications like fermentation and bioremediation, optimizing the growth yield coefficient is essential. Researchers and engineers aim to maximize microbial biomass production while minimizing substrate consumption. A high Y value indicates efficient biomass production from available resources.
2. Environmental Applications
In environmental microbiology, the growth yield coefficient is used to assess the effectiveness of microbial processes in remediation, such as wastewater treatment. It helps determine how efficiently microorganisms can degrade pollutants or remove contaminants from the environment.
3. Biopharmaceutical Production
In the production of biopharmaceuticals using microbial hosts, such as bacteria or yeast, the growth yield coefficient influences product yield. Higher Y values mean more biomass, which can translate into increased product formation.
4. Microbial Ecology
In microbial ecology studies, Y is employed to understand microbial interactions in ecosystems. It provides insights into which microorganisms are more efficient in utilizing available substrates and competing for resources.
5. Nutrient Management
In agriculture and soil microbiology, the growth yield coefficient can be used to evaluate nutrient utilization by soil microorganisms. This knowledge can inform sustainable agricultural practices and soil management.
Factors Affecting Y
Several factors can influence the growth yield coefficient, including the type of microorganism, the specific substrate used, environmental conditions (e.g., temperature and pH), and the presence of inhibitors or co-substrates. Researchers often conduct experiments to determine the Y value for a particular microbial system under specific conditions.
Conclusion
The growth yield coefficient, denoted as Y, is a valuable parameter that quantifies the efficiency of microbial growth in converting substrate into biomass. Its significance spans various fields, from biotechnology and environmental science to agriculture and pharmaceutical production. Understanding and optimizing Y allows researchers and industries to harness the potential of microorganisms efficiently and sustainably, making it a fundamental concept in microbiology and biotechnology.
