The biopharmaceutical industry, defined by its precision and adherence to regulatory standards, cannot afford errors or inconsistencies. However, deviations—events where processes or procedures deviate from approved standards—are inevitable given the complexity of operations. Addressing these deviations systematically is critical to ensuring product quality, patient safety, and regulatory compliance.
This article delves into the essentials of deviation handling, covering definitions, types, a detailed management process, and multiple real-world case studies to illustrate the importance of effective deviation management.
What is a Deviation in Biopharma?
Table of Contents
ToggleIn biopharma, a deviation is any occurrence that strays from approved protocols, procedures, or specifications during manufacturing, testing, or documentation. It represents a departure from established processes and must be promptly identified, documented, and resolved.
Common Examples of Deviations:
- Sterilization failure in aseptic environments.
- Discrepancies in equipment calibration.
- Deviations in environmental conditions (e.g., temperature excursions).
- Incorrect documentation or labelling of materials.
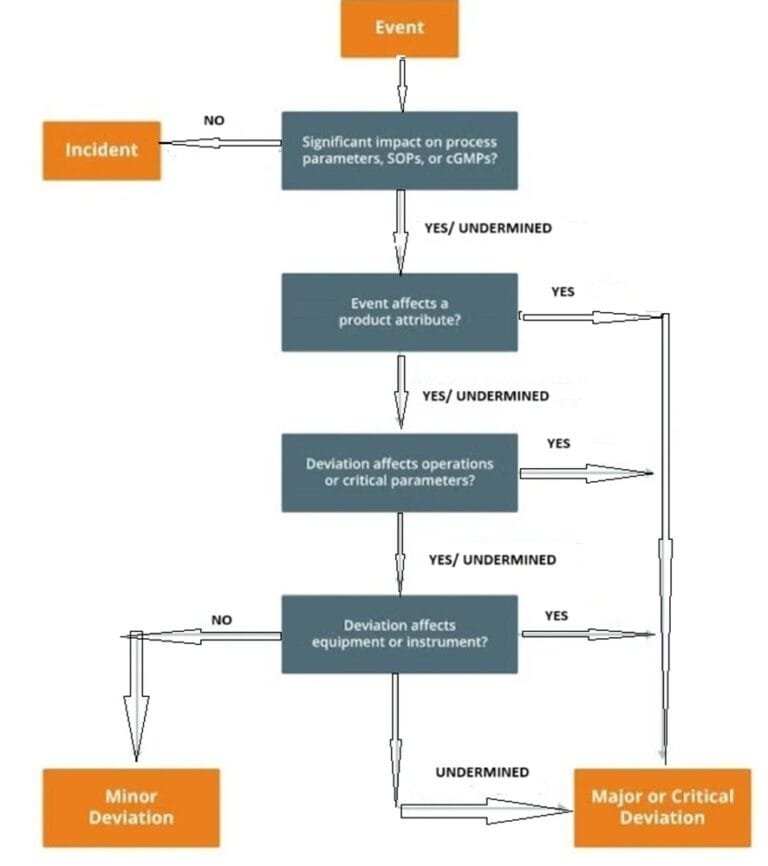
Types of Deviations
Critical Deviation
Critical deviations are high-risk events that directly impact product safety, quality, or regulatory compliance.
Example: Presence of microbial contamination in a sterile environment.
Major Deviation
Major deviations can potentially affect product quality or compliance but do not immediately threaten patient safety.
Example: Failure to maintain temperature requirements during transportation of biopharma products.
Minor Deviation
Minor deviations are low-risk and unlikely to affect product quality or compliance.
Example: A typo in standard operating procedures (SOPs) that does not impact actual processes.
Planned Deviation
Planned deviations are intentional, pre-approved temporary changes to procedures.
Example: Using an alternative supplier for a raw material due to supply chain disruptions.
Unplanned Deviation
Unplanned deviations occur unexpectedly and without prior approval.
Example: A sudden power outage during an upstream fermentation process.
Deviation Handling Process: Step-by-Step Guide
Effective deviation management involves a structured approach. Here’s how deviations should be handled:
Identification and Reporting
Deviations must be identified and reported immediately. Operators and supervisors should be trained to recognize deviations in real-time.
Documentation
All deviations must be thoroughly documented in a deviation report. Information to include:
- Deviation description.
- Date, time, and location.
- Immediate corrective actions.
- Personnel involved.
Risk Assessment
Classify the deviation as critical, major, or minor based on its potential impact. This helps prioritize resources for investigation and resolution.
Root Cause Analysis (RCA)
Investigate the deviation’s underlying cause using tools like the 5 Whys, Fishbone Diagram, or Fault Tree Analysis.
Corrective and Preventive Actions (CAPA)
Corrective Actions:
Resolve the immediate issue.
Preventive Actions:
Implement measures to avoid recurrence.
Review and Approval
The deviation report and CAPA plan should be reviewed and approved by the Quality Assurance (QA) team.
Closure
Ensure all actions are implemented and their effectiveness validated before closing the deviation.
Case Study 1: Microbial Contamination in Upstream Fermentation
Scenario
A biopharma company detected microbial contamination during the fermentation stage, indicated by abnormal pH levels in the bioreactor.
Actions Taken
- The batch was quarantined, and production was halted.
- RCA revealed incomplete sterilization due to a malfunction in the autoclave.
Corrective and Preventive Actions (CAPA)
- Discarded the contaminated batch and re-sterilized the bioreactor.
- Revised SOPs for autoclave maintenance.
- Installed automated sterilization cycle monitors.
Case Study 2: Temperature Excursion During Transportation
Scenario
A shipment of a temperature-sensitive vaccine experienced a temperature excursion beyond the allowable range during transportation.
Investigation
- The deviation was classified as major.
- RCA revealed that the data logger in the transport container malfunctioned, causing an undetected temperature rise.
Corrective and Preventive Actions (CAPA)
- Rejected the affected shipment.
- Procured new data loggers with automated alarm systems.
- Conducted training for logistics staff on proper container handling.
Case Study 3: Equipment Calibration Failure
Scenario
A biopharmaceutical manufacturing plant discovered during routine operations that one of the critical bioreactors was overdue for calibration.
Investigation
- RCA determined that the maintenance schedule had been overlooked due to a scheduling error in the equipment management software.
- The deviation was classified as a major deviation.
Corrective and Preventive Actions (CAPA)
- Recalibrated the bioreactor and verified its functionality before resuming operations.
- Updated the equipment management system to include automated reminders for calibration deadlines.
- Assigned a dedicated maintenance coordinator to oversee equipment schedules.
Case Study 4: Documentation Error in Batch Records
Scenario
During a quality control review, it was discovered that a batch record contained inconsistent data for a critical process parameter.
Investigation
- The deviation was classified as minor since the error did not impact product quality or compliance.
- RCA found that the operator failed to double-check entries due to insufficient training.
Corrective and Preventive Actions (CAPA)
- Corrected the documentation error and verified accuracy through cross-referencing.
- Enhanced operator training programs on proper record-keeping practices.
- Introduced an additional layer of review for critical documents.
Why Deviation Management is Critical in Biopharma
Regulatory Compliance
Deviation handling ensures adherence to strict guidelines set by regulatory agencies like the FDA, EMA, and WHO.
Product Integrity
Timely resolution of deviations prevents compromised products from reaching the market.
Continuous Improvement
Deviation investigations highlight process inefficiencies, enabling continuous improvement.
Audit Readiness
Thorough documentation of deviation management demonstrates control and transparency during inspections.
Conclusion
In the biopharma industry, deviations are not just operational hiccups—they are opportunities for growth and improvement. By adopting a robust deviation management process, companies can safeguard product quality, enhance compliance, and build resilience into their operations.
The real-world case studies provided here demonstrate how effective deviation handling can prevent critical risks and improve overall efficiency. With the right systems, tools, and culture, organizations can transform deviations into milestones of success.
What are your strategies for managing deviations? Share your experiences and insights below!
